Publikationen
S. Samberger, I. Weißensteiner, M. A. Tunes, L. Stemper, C. Kainz, R. Morak, P. J. Uggowitzer, and S. Pogatscher; Journal of Manufacturing Processes; 2025
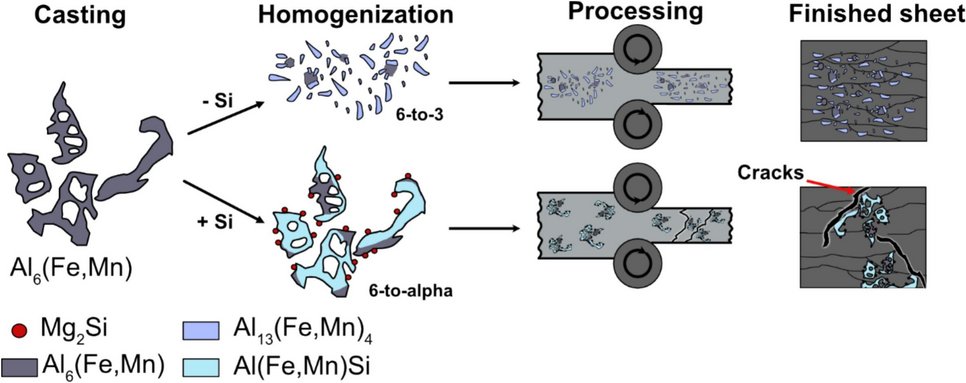
Aluminum crossover alloys offer a broad property profile within a single composition, but due to the growing demand for recycling in the aluminium industry, they will be required to mitigate the impact of tramp elements such as Fe and Si. This study investigates the influence of Fe/Si ratios and cooling rates during solidification on phase transformations and microstructure evolution in AlMgZn(Cu) crossover alloys, aiming to increase recycling content and maintain processability. Thermodynamic simulations, coupled with experimental validation, reveal two critical phase transformations during homogenization: the 6-to-3 transformation (Al6(Fe,Mn) → Al13(Fe,Mn)4) and the 6-to-α transformation (Al6(Fe,Mn) → Al(Fe,Mn)Si). These transformations are governed by the Fe/Si ratio and cooling rate, significantly affecting intermetallic phase morphology. The 6-to-3 transformation can effectively decrease the size of intermetallic particles, facilitating processability in relevant industrial conditions. Higher cooling rates upon solidification (≈60 K/s) always result in small, spheroidized phases, ensuring rollability. In contrast, slow cooling rates (≤1 K/s) often promote coarse, stable phases that hinder processability. However, at cooling rates around 3 K/s the intermetallic phase morphology highly depends on the Fe/Si ratio. When Fe and Si levels are simultaneously high, the 6-to-α transformation yields hard-shell/soft-core structures that impair mechanical integrity, while a higher ratio governs a beneficial 6-to-3 transformation. This study provides new insights into impurity-induced phase transformations and their role in determining processability in industrially relevant conditions. By linking microstructural control to sustainable alloy design, the results serve as a foundation for the development of crossover aluminum alloys optimized for high scrap content.
P. Aster, P. Dumitraschkewitz, P. J. Uggowitzer, I. Weißensteiner, M. A. Tunes, F. Schmid, L. Stemper, and S. Pogatscher, Materials & Design 114341 (2025)
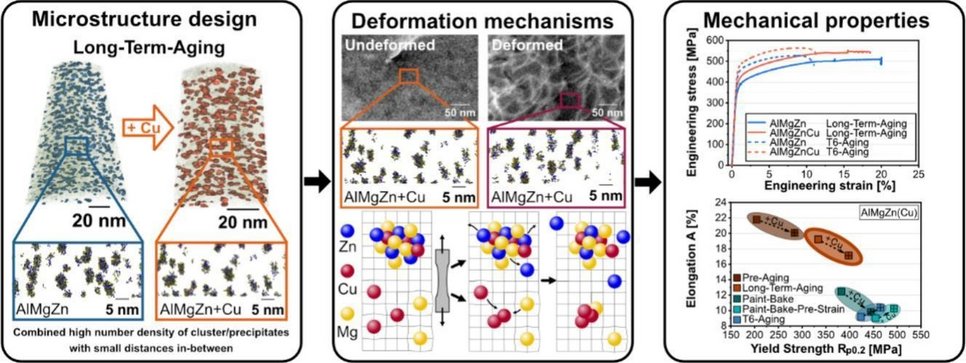
This study investigates clustering and precipitation in Al-Mg-Zn-(Cu) crossover alloys, focusing on Cu’s role and the long-term aging (LTA) process. LTA at 60 ◦C for 42 days promotes the formation of a dense cluster/precipitate structure, significantly enhancing strain hardening while maintaining high elongation. This treatment achieves an optimized balance of yield strength (~400 MPa) and elongation (~17 %), outperforming conventional aging methods such as pre-aging, and paint baking. The addition of Cu plays a critical role by pro moting higher cluster number densities, refining spatial distribution, and influencing chemical compositions. Cu effectively hinders dislocation motion, thereby increasing yield strength, and alters strain-hardening behavior by impeding dynamic recovery and reducing dislocation annihilation. Unlike in 6xxx-series alloys, no strain-induced clustering occurs in LTA state, with partial redissolution observed for some elements. Detailed analysis of strengthening mechanisms reveals the need for precise evaluation of individual cluster types and compositions to fully understand relationships between cluster volume fraction, size, spacing, and mechanical properties. While clustering behavior is well-studied in 2xxx, 6xxx and 7xxx-seriesalloys, research in Al-Mg-Zn-Cu crossover alloys remains comparatively underdeveloped. This work provides new insight into clustering in crossover alloys and demonstrates LTA as a promising route to unlock their full mechanical potential.
Abgeschlossene Arbeiten
Masterarbeit von Thomas Wachter
Alternative battery systems, such as rechargeable aluminum batteries (RABs), offer advantages over state-of-the-art lithium-ion batteries, especially for large-scale energy storage applications. Aluminum is abundant, inexpensive, and provides high gravimetric energy density. In the past, the importance of the aluminum anode for cycling stability in non-aqueous RABs has often been underestimated. With recent advances in cathode performance, a shift towards anode-focused research has emerged. However, the native oxide layer of aluminum causes challenges such as inhomogeneous corrosion, passivation, and unstable plating/stripping behavior at high current densities. Most studies concentrate on modifying, substituting, or removing the oxide layer and on suppressing dendrite growth. It has been reported that zinc alloying effectively increases battery performance by altering the native oxide in Al-air batteries. To test whether this observation also applies to battery systems employing non-aqueous electrolytes, anodes of Al 3.5 wt.% Zn were produced and subjected to symmetric cell tests, which revealed increased overpotential. Besides the effect of the chemical composition, the influence of surface roughness on battery performance was assessed as well. Subsequently, the focus was placed on aluminum-magnesium alloys with controlled microstructure. A combined approach is investigated, in which oxide stability is modified through alloying while a porous-like surface is generated through selective etching of a homogeneously distributed secondary phase. Anodes from two alloys (Al-6Mg-0.4Mn-0.2Zr-0.2Sc and Al-6Mg-0.4Mn-0.3Zr-0.08Y) with tailored heat treatments were produced, characterized using SEM/EDX, and evaluated in symmetric cell tests employing Al3Cl/[EMIm]Cl as electrolyte. A significant increase in effective surface area could be achieved by selective etching of magnesium-enriched islands using phosphoric acid. The introduced alloying elements, as well as trace impurities from the commercially pure aluminum base, did not induce cycling instability at current densities of 0.05 mA cm-2 and 0.1 mA cm-2. Compared to 99.99% pure aluminum foil, which is the current state-of-the-art in literature, the alloyed anodes exhibit a reduction in initial charge-transfer resistance of 88% and 77% for the scandium- and yttrium-containing samples, respectively, and achieved stable cycling more rapidly. Electrochemical impedance spectroscopy measurements after cycling revealed only negligible differences in charge-transfer resistance. Further studies are required to evaluate the dendrite-suppression capability at higher current densities and in a full-cell configuration.
