A fundamental mineralogical investigation of downhole cements within the context of Underground Hydrogen Storage
VIDEO
coming soon...
Introduction
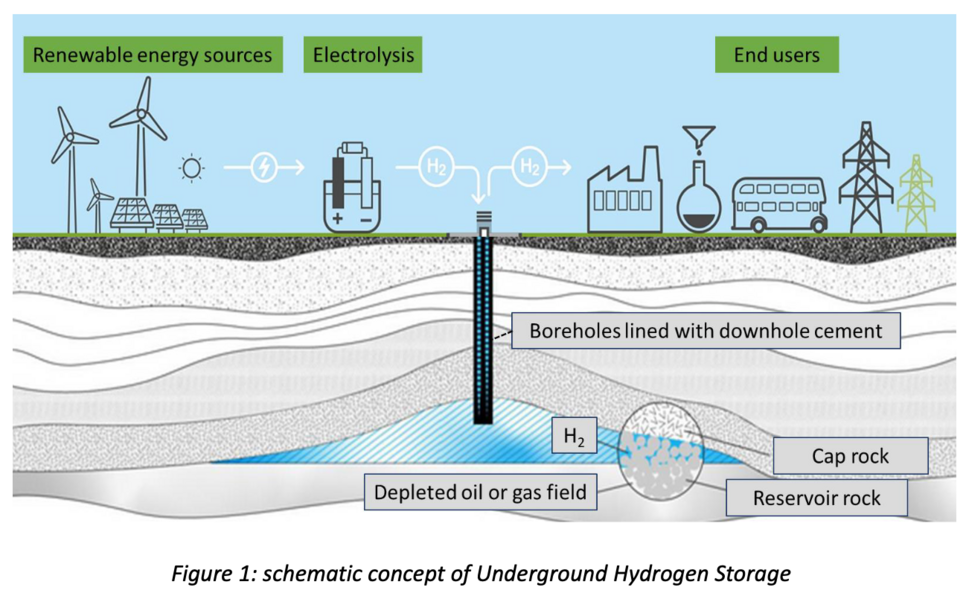
Hydrogen is nowadays commonly considered a promising way of storing energy from renewable energy sources, hence increasing their efficiency. Underground Hydrogen Storage (UHS) e.g., the idea of using natural geological bodies in the underground like aquifers, salt caverns or depleted oil and gas fields, promises great potential due to vast storage capacities (Fig. 1). However, to make UHS a feasible process, fundamentals research investigating not just the integrity of reservoir and cap rocks, but also downhole cements applied in boreholes during the storage life of the well against hydrogen is essential. This is particularly important once the injection scale is industrial, as there is a sever knowledge gap regarding the effect hydrogen might have on the mineralogical phase composition and subsequently on physical and mechanical parameters of downhole cement, which is a significant element for the well integrity and therefore safety.
Methodology
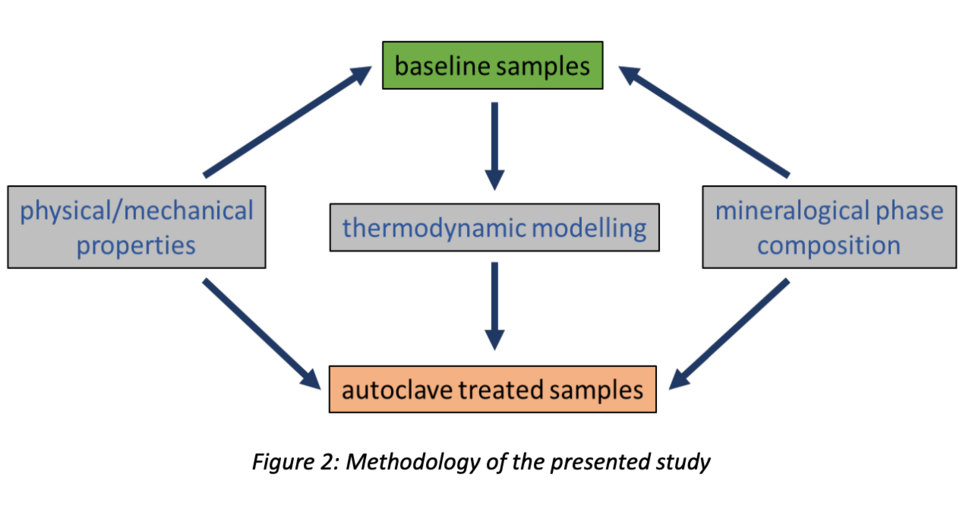
The presented study is an interdisciplinary approach among geoscientists and drilling engineers trying to link potential hydrogen induced changes in the mineralogical phase composition of downhole cements to changes in physical and mechanical properties (Fig. 2). Therefore, applied mineralogical methods are: XRD, FE-SEM and EPMA. The physical and mechanical properties investigated are
porosity, pore size distribution and permeability, using Hg-porosimetry, N2 sorption and nitrogen permeation as well as compressive and tensile strength, respectively. The rationale of the investigations is to compare non-treated samples (baseline) with those exposed to hydrogen for 3 - 4 weeks at well-defined p & T conditions using hydrothermal autoclaves. Additionally, thermodynamic modelling was performed using the software GEMS (Gibbs Energy Minimization Selector) to evaluate whether a hydrogen-induced reaction in cements at thermodynamic equilibrium is possible at all.
Results
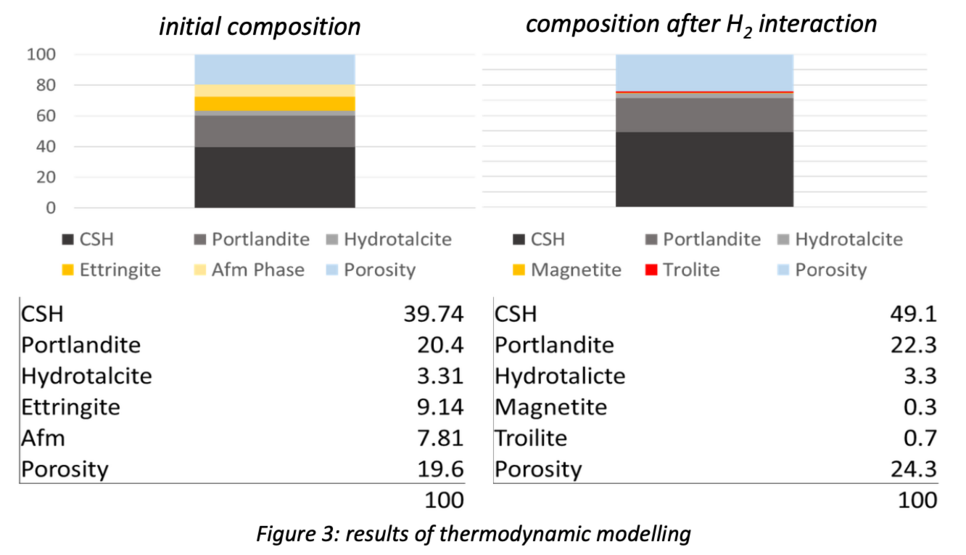
Thermodynamic modelling (Fig. 3) indicates that certain redox-sensitive phases within hardened downhole cement are susceptible to hydrogen alteration caused by the strong reducing character of hydrogen. Especially ferric iron and sulphate bearing phases like brownmillerite, monosulfoaluminate (AFm) and ettringite (AFt) are altered, resulting in the formation of magnetite and iron sulphides.
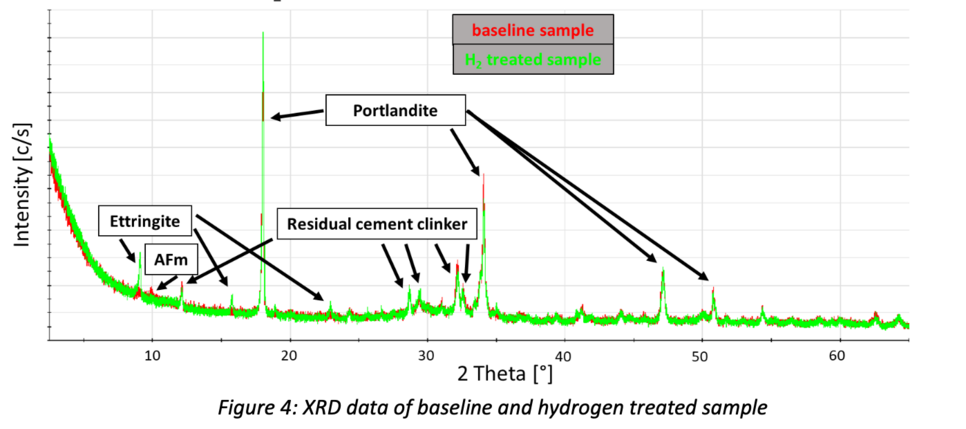
X-ray Diffraction (XRD) measurements indicate the absence of AFm phase and the formation of ettringite within the hydrogen treated sample (Fig. 4). According to the results of thermodynamic modelling, ettringite should also be absent. This contradiction is not yet fully understood. Maybe thermodynamic equilibrium was not reached during H2 treatment.
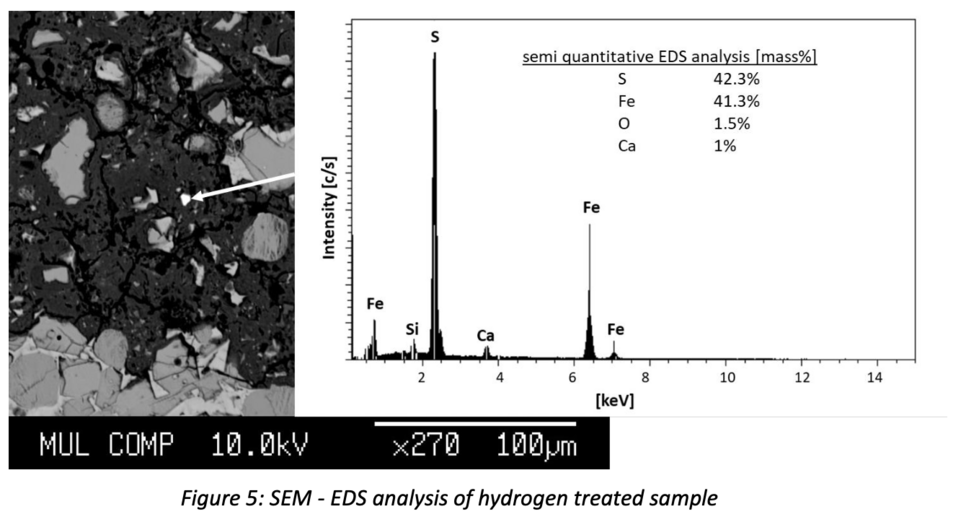
SEM-EDS analysis show the formation of iron sulphides within the H2 treated sample (Fig. 5). Even though only minor amounts of small (<3 µm) grains are found, this confirms the results of thermodynamic modelling.
Conclusion
- Thermodynamic modelling indicates hydrogen induced changes in the mineralogical phase composition of downhole cements.
- SEM-ES analysis confirms the formation of iron sulphides as indicated by modelling.